Since November of 2020, any Israeli company wishing to export cannabis has first been required to work with Panaxia.
By IDAN ZONSHINE
April 15, 2021 | JPost

As companies in several fields have struggled to stay afloat during the economic crisis brought on by the coronavirus, one company has been seeing the exact opposite effect in recent months.Israeli medical cannabis giant Panaxia on Sunday reported an estimated record growth of at least NIS 19 million in revenue for the first fiscal quarter of 2021, a massive 56% growth compared to the corresponding quarter last year, which saw approximately NIS 12.2m. in growth.
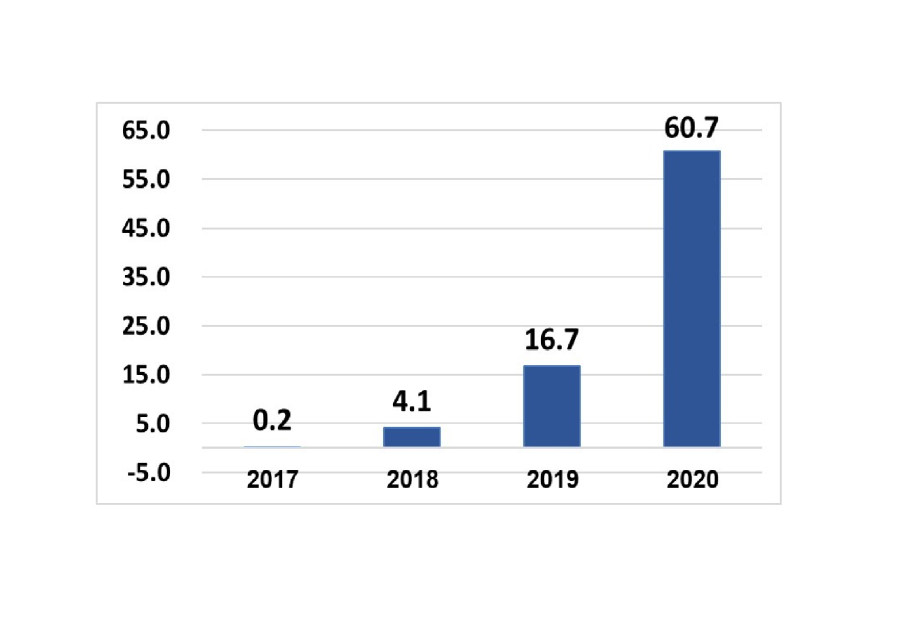
Panaxia’s revenues have continued to grow progressively throughout the past year, with the company seeing a 12% growth this quarter when compared to the final quarter of 2020, which amounted to approximately NIS 17m.
Panaxia CEO Dadi Segal said in a statement that Panaxia was “proud to close out the first quarter of 2021 with record revenues and double-digit growth compared to the previous and corresponding quarter the previous year.”However, this meteoric rise in revenue does not seem to have happened due to a rise in demand for medical cannabis, as several major Israeli companies have seen dire financial losses in the past year.
The discrepancy also cannot be chalked up to a specific rise in demand for Panaxia’s products which, while they are definitely considered a high benchmark in regard to quality, do not seem to hold any major technological edge which would set them apart so vastly from their many qualified competitors.The reason for Panaxia’s success seems to lie in their regulatory planning, seeing as last November, they became the first – and, so far, only – Israeli company to receive the Health Ministry’s approval to commercially export medical cannabis abroad.“This quarter’s results are the initial results of the implementation of Panaxia’s export strategy, with the commencement of sales of our premium products in Germany and Cyprus,” Segal said.Since then, they have begun exporting medical cannabis products overseas to Australia, Germany, France and Cyprus, as the rest of Israel’s cannabis market has continued to lag behind.For example, another of Israel’s largest medical cannabis companies, Seach Medical Group, announced a new export deal to Australia on Sunday, agreeing to supply an impressive 2 tons of cannabis flowers to the Australian company Cannatrek through the end of 2022.However, in order to sign this export deal, Seach first needed to partner with Panaxia to ensure that their products met standards required by Panaxia’s EU-GMP (good manufacturing procedures) license, which differ vastly from the standards required for the IMC-GMP license needed to sell medical cannabis in Israel.
The discrepancy also cannot be chalked up to a specific rise in demand for Panaxia’s products which, while they are definitely considered a high benchmark in regard to quality, do not seem to hold any major technological edge which would set them apart so vastly from their many qualified competitors. The reason for Panaxia’s success seems to lie in their regulatory planning, seeing as last November, they became the first – and, so far, only – Israeli company to receive the Health Ministry’s approval to commercially export medical cannabis abroad.“This quarter’s results are the initial results of the implementation of Panaxia’s export strategy, with the commencement of sales of our premium products in Germany and Cyprus,” Segal said. Since then, they have begun exporting medical cannabis products overseas to Australia, Germany, France, and Cyprus, as the rest of Israel’s cannabis market has continued to lag behind. For example, another of Israel’s largest medical cannabis companies, Seach Medical Group, announced a new export deal to Australia on Sunday, agreeing to supply an impressive 2 tons of cannabis flowers to the Australian company Cannatrek through the end of 2022. However, in order to sign this export deal, Seach first needed to partner with Panaxia to ensure that their products met the standards required by Panaxia’s EU-GMP (good manufacturing procedures) license, which differ vastly from the standards required for the IMC-GMP license needed to sell medical cannabis in Israel.

Assi Rotbart, GM and co-founder of Panaxia spoke with The Jerusalem Post on Monday to explain how Panaxia became the sole holder of an EU-GMP license in Israel’s medical cannabis industry.“The standards required by the EU-GMP and the IMC-GMP are so different that you can’t take one, make a few adjustments and work with both,” he said. “In order to keep an IMC-GMP and an EU-GMP we are required to maintain two quality control systems at the same time.”He said that the process to build two separate regulatory systems for the company was devised around two years beforehand – before the IMC-GMP standards were even devised – due to a great deal of stable data collection and resources which the company knew such a project would demand. Rotbart told the Post that while each licensing system has its strengths, they are both good measures of quality, effectiveness, and safety. However, while the EU-GMP is recognized by many countries worldwide, even showing several similarities with the Israeli GMP – which exists for medical products which are not cannabis-related – the IMC-GMP is solely applicable to Israeli companies, forcing companies to choose between either having to spend excess time and resources on maintaining two vastly different regulatory systems at once or drop the idea of exporting cannabis altogether. However, while the EU-GMP is recognized by many countries worldwide, even showing several similarities with the Israeli GMP – which exists for medical products which are not cannabis-related – the IMC -GMP is solely applicable to Israeli companies, forcing companies to choose between either having to spend excess time and resources on maintaining two vastly different regulatory systems at once, or drop the idea of exporting cannabis altogether.
